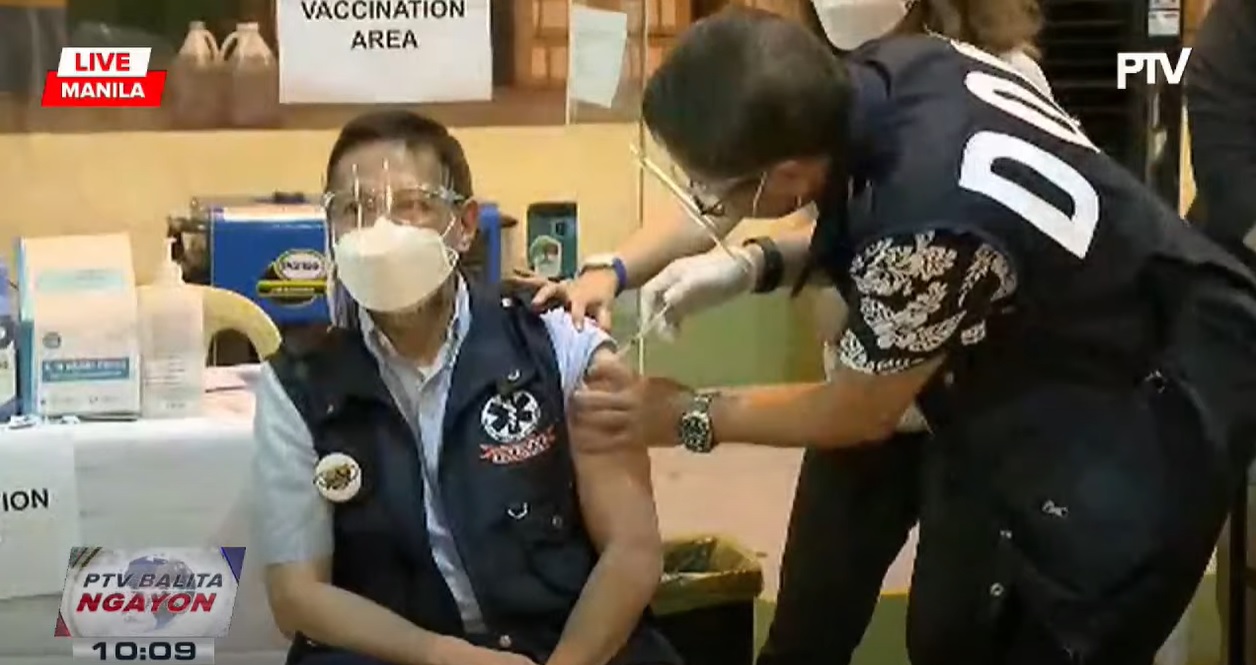
Health Secretary Francisco Duque has received his first dose of Chinese-made Coronavac vaccine from the company Sinovac.
“To further assure vaccine safety and reiterate the importance of vaccines in the fight against the pandemic, Secretary Duque—after waiting for his turn to get vaccinated—also received his first jab of the CoronaVac vaccine as part of the Priority Group A2 (senior citizens),” the DOH said in a statement.
“As I receive my dose of the COVID-19 vaccine today, I invite everyone to do the same, and choose to be protected. Let us all take part in protecting public health, and let us be in unison in spreading one message: that vaccines are safe, and vaccines are effective,” Sec. Duque said.
RELATED STORY: Philippines expects steady COVID-19 vaccine supply starting June
The DOH and the Philippine Food and Drug Administration (FDA) today assured the public that COVID-19 vaccines are safe and effective in preventing hospitalization and deaths from the disease, and that the benefits of vaccination far outweigh its risks.
The country has so far inoculated over one million Filipinos. Based on the monitoring of the Adverse Events Following Immunization (AEFI) presser vaccinees who experienced very mild side effects, and only 1.5% of the total AEFIs were serious.
READ ON: Philippines grants emergency use of J&J, Bharat Biotech COVID-19 vaccines
The DOH and FDA reported that all reported serious AEFIs were later found to be coincidental and totally unrelated to the vaccine after careful and thorough causality assessment.
“From the start of our vaccination period to April 10, only 1.5% of the total AEFIs were serious cases. This means that for every 1,000,000 vaccinated, less than 0.04% may experience serious AEFI cases. But it does not necessarily mean that the vaccine causes the events. This risk is much lower than the fatality of the COVID-19 infection in our country which has a 1.69% fatality rate,” FDA Director General Rolando Enrique Domingo said.