The Food and Drug Administration (FDA) has issued an advisory informing consumers in the Philippines that fake Paracetamol Biogesic 500 mg tablets are currently being sold in the market.
The FDA was able to determine that some of the tablets being sold are indeed fake, with the verification of the market authorization holder, United Laboratories, Inc.
The FDA further reminded the public not to consume these counterfeit medicines as they can possibly cause dangerous side effects, and that consumers should purchase drug products only from FDA-licensed establishments.
The agency also reminded drug stores and pharmacies that selling fake medicines can also be penalized as doing such act is a direct violation of Republic Act 9711 or the Food and Drug Administration Act of 2009, and Republic Act 8203 or the Special Law on Counterfeit Drugs.
The FDA has also released photos that will help the public differentiate the authentic tablets from its counterfeit.
As seen on the photos, the real and fake tablets have noticeable differences in terms of color, printed information, and packaging.
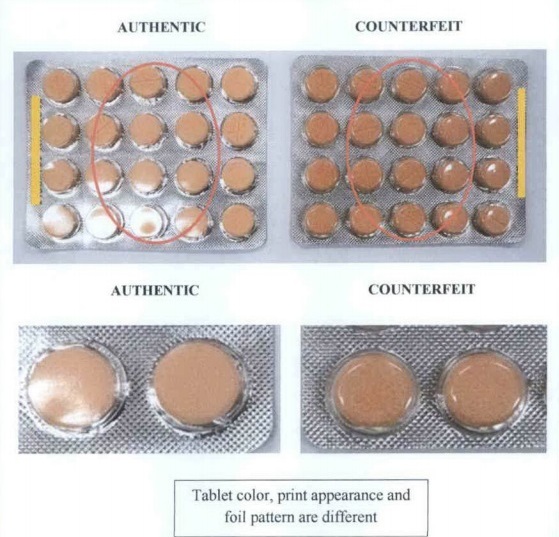
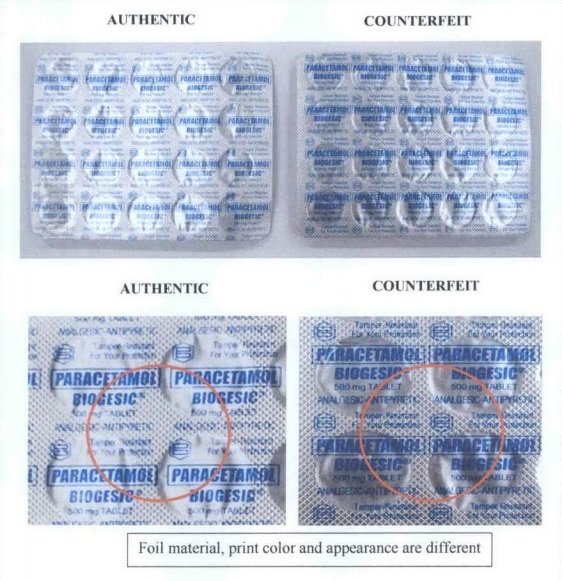
Biogesic’s manufacturer, Unilab, has also warned the public against the fake tabled and even said that “round Biogesic 500mg tablets are no longer being manufactured nor sold.”
Unilab said that Biogesic now “comes in new packaging and have a caplet form.”



